About us
The Panel on Research Ethics is responsible for addressing the evolving needs of Canada's three federal research agencies, CIHR, NSERC and SSHRC, in promoting the ethics of research involving humans.
Panel Members
Panel on Research Ethics (PRE)
The Panel on Research Ethics (PRE) is composed of 12 members drawn from across the country to represent a wide spectrum of expertise and experience in the ethics of human research, such as research involving Indigenous peoples, ethics and ethics review, research administration, research in the health, natural and social sciences, humanities and engineering, law, as well as a lay perspective.
Working in collaboration with and drawing support from expert groups, PRE will add to the substantial base of ethics knowledge and ability that already exists in Canada.
The members of PRE are:

Conrad Fernandez (Chair)
Professor and Head
Division of pediatric hematology/oncology
Department of Pediatrics
IWK Health Centre
Dalhousie University

Susan Babcock
Independent consultant

Kelly Bannister
Co-Director of the POLIS Project on Ecological Governance
Centre for Global Studies
University of Victoria

Khaled El Emam
Canada Research Chair in Medical Artificial Intelligence
University of Ottawa

Sean Hillier
Associate professor and York Research Chair in Indigenous Health Policy & One Health
Faculty of Health
York University

Marc Joanisse
Professor, Department of Psychology and Western Institute for Neuroscience
Associate Dean Research, Faculty of Social Science
University of Western Ontario

Edwin Laryea
Community Representative, Research Ethics Board
University of Waterloo

Anik Nolet
Senior Research Ethics Advisor
Centre intégré en santé et services sociaux du Centre-Sud-de-l’Île-de-Montréal

Wendy Rodgers
President and Vice-Chancellor
University of Prince Edward Island

Alice Virani
Director of the Clinical Ethics Service
Provincial Health Service Authority
Clinical Assistant Professor
Department of Medical Genetics
University of British Columbia

Nancy Walton
Associate Dean, Student Affairs, Yeates School of Graduate Studies
Toronto Metropolitan University

Ma’n Zawati
Associate Professor, Faculty of Medicine, McGill University
Research Director, Centre of Genomics and Policy
Ex-officio Member
Executive Director, Secretariat on Responsible Conduct of Research
Former Panel Members
- Judith Bartlett
- Michel Bergeron
- Bill Bogart
- Pierre Boulos
- Marie-Pierre Bousquet
- Howard Brunt
- Julie Bull
- Marlene Brant Castellano
- Laurie Chan
- Peter Chow-White
- Bruce Clayman
- Timothy Caulfield
- Annabelle Cumyn
- Édith Deleury
- Pierre Deschamps
- Hubert Doucet
- Carolyn Ells
- Lawrence Felt
- Deborah Fels
- Pierrette Fortin
- Jim Frideres
- Norman Frohlich
- Lisa Given
- Joyce Helmer
- Paul Johnston
- Patricia Kosseim
- James Lavery
- Ndiaga Loum
- Samuel Ludwin
- Diane Martz
- Daphne Maurer
- Ian Mitchell
- Patrick O'Neill
- Florence Piron
- Gordon Robinson
- Carol Sawka
- Martin Schechter
- Janice Singer
- Maureen Smith
- Veronica Stinson
- Susan Sykes
- Lehana Thabane
- Will van den Hoonaard
- Peter Venner
- Brent Windwick
Organizational Structure
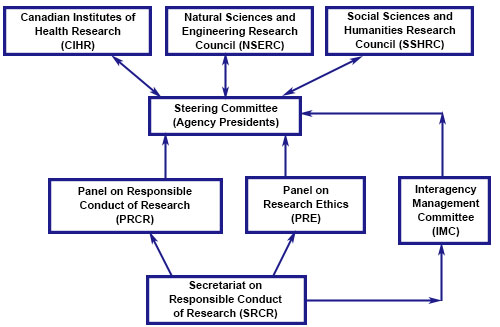
Organizational Structure - long description
The Secretariat on Responsible Conduct of Research supports the Advisory Panel on Research Ethics and the Advisory Panel on Responsible Conduct of Research on policy matters; and reports to the Interagency Management Committee (IMC) on interagency administrative and operational matters.
The IMC reports to a Steering Committee, composed of the Agencies' Presidents, on operational and strategic issues, as appropriate and required.
Terms of Reference
Mandate
Panel on Research Ethics (PRE) is responsible for addressing the evolving needs of the three Agencies in promoting the ethics of research involving humans. It functions as an interdisciplinary and pluralistic advisory body, providing the Agencies with independent reflection and advice on human research ethics, consistent with the Tri-Council Policy Statement: Ethical Conduct of Research Involving Humans, 2nd edition (TCPS 2).
PRE’s mandate is to:
- promote high ethical standards of conduct in research involving humans;
- advise the Agencies about the ongoing development and evolution of the TCPS 2;
- establish or commission ad hoc expert groups to address specific issues;
- provide interpretations of the TCPS 2 for its implementation and use;
- learn from and respond to evolutions in research ethics issues and practices in a national and international context;
- promote and support the implementation of the TCPS 2;
- identify educational activities and mandate the Secretariat to implement and promote them;
- participate in regional or national discussions related to human research ethics and the research ethics review process;
- recognize the diversity of approaches used in research involving humans;
- report annually on its activities to the Presidents of the Agencies.
In carrying out its mandate, PRE is committed to openness and transparency.
Membership
PRE has 12 members. In addition, the Executive Director of the Secretariat and a representative from CIHR's Stem Cell Oversight Committee are ex officio members without voting rights. Observers may also be invited to participate in the meetings.
In addition to geographical and gender representation, PRE membership provides:
- a balanced representation of researchers in biomedical and health sciences, social sciences and humanities, and those in the natural science and engineering fields undertaking research involving humans;
- expertise or experience in ethics, law, REB operations and research administration at an institutional level;
- representation from the Indigenous community and research participants.
All members are volunteers. They do not receive honoraria in compensation for their contribution of expertise and time, though the Secretariat covers their expenses (such as travel and accommodation).
Terms
The Presidents of the three Agencies appoint PRE members. Initial appointments are for a period of three years, with the possibility of extension for one to three years for a maximum total of six years. The appointments are staggered to provide flexibility and continuity.
PRE’s Chair is also appointed by the three Presidents. The initial term of appointment is for two years with the possibility of one or more extensions.
Meetings
PRE will usually meet at least once each year, communicating further in person or by teleconference as required. The Executive Director and staff of the Secretariat participate in the meetings.
Quorum
A majority of its members (50% plus 1), which must include the Chair, or his/her designate.
Reporting Structure
PRE reports to the Presidents of the Agencies, providing recommendations regarding the TCPS 2. The Presidents then determine the appropriate action to be taken. The Panel Chair reports annually on PRE's activities to each of the Agencies' Councils.
Reference
Tri-Council Policy Statement: Ethical Conduct of Research Involving Humans, 2nd edition
- Date modified: